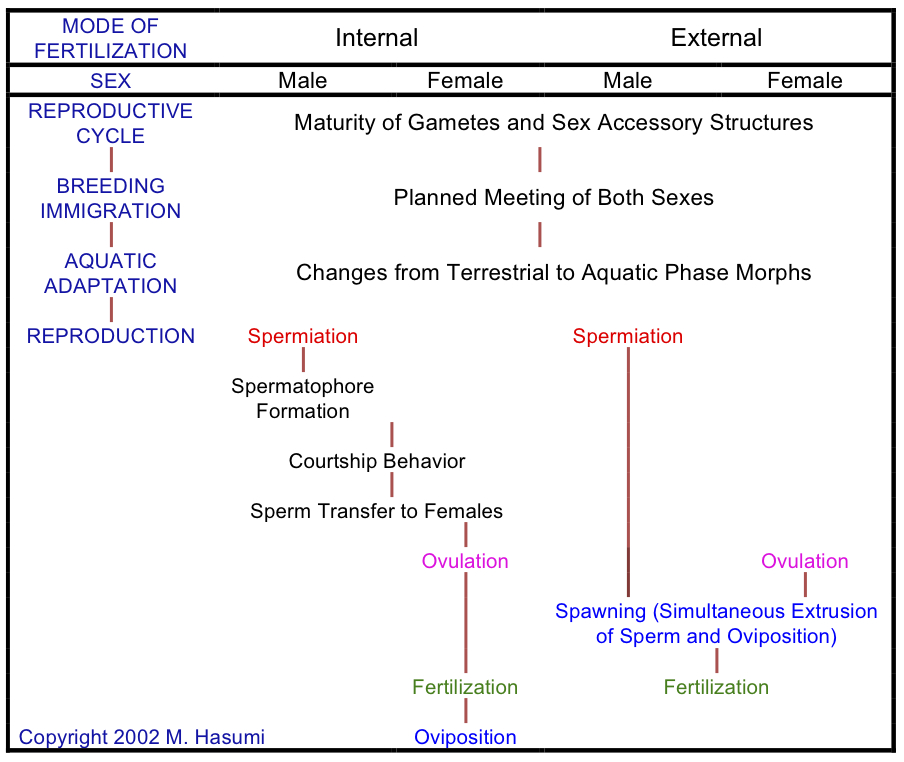
(1) As previously stated, they practice "external fertilization" by means of "spawning" (i.e., simultaneous extrusion of sperm and oviposition) in water during the breeding season, but a male of Ranodon sibiricus with external fertilization may produce a single large spermatophore, in which a female deposits a pair of egg sacs on the spermatophore that was previously deposited by a male in a mountain brook (see Paraskiv 1953; Nussbaum, 1985; Pough et al., 2001). On the other hand, of this species, Thorn (1994) observed a similar behavior to that of Hynobius retardatus (i.e., typically stereotyped spawning behavior that is common to hynobiids: for a review, which has discussed on the absence or presence of spermatophores, see Hasumi [2015]: doi: 10.1007/s10211-015-0214-z). By the way, Pough et al. (2015) still have described that a male of R. sibiricus produces a spermatophore.
(2) They breed in aquatic habitats, after awaking from hibernation and then immigrating from land to water during spring (the family Plethodontidae, which corresponds to approximately 70% of all urodeles, includes completely terrestrial species throughout the life, and ambystomatids and salamandrids with internal fertilization are counterparts of hynobiids among migratory salamanders), but a lotic-breeding salamander Hynobius kimurae immigrates from land to water for aquatic hibernation annually during fall (Kakegawa et al., 2017: doi: 10.1016/j.jcz.2017.04.003; Kakegawa and Hasumi, 2017: doi: 10.1002/rra.3162; Kakegawa and Hasumi, 2018: doi: 10.1111/azo.12222).
(3) A female deposits a pair of egg sacs, including a full clutch of unfertilized eggs (in urodeles with internal fertilization, a female deposits fertilized eggs one by one in many places as clumps or non-clumps, e.g., Taricha deposit their eggs in clumps, whereas Triturus lay their eggs individually; Arntzen, 2002).
(4) Males during scramble competition focus on a pair of egg sacs, instead of a female during and after oviposition (Hasumi, 1994: doi: 10.2307/1564635; Hasumi, 2001: Stable URL). In contrast, in urodeles with internal fertilization, males recognize a female as a limited resource (Pough et al., 2001, 2015).
(5) Because hynobiid salamanders are not known to exercise any form of "parental care" (i.e., egg-guarding primarily against predators in aquatic development), regardless of lentic or lotic habitats, both Dr. Tamotsu Kusano and I do not agree with Nussbaum's (1985) citation of 2 lotic-breeding species of hynobiids as evidence for parental care in this family (in comparison, e.g., male Andrias japonicus guards eggs in his den, and female plethodontid salamanders brood their eggs deposited on land as terrestrial nesting). Hasumi (2015) provided a focal review that parental care has not yet been demonstrated in any species of the family Hynobiidae and then provided valid alternatives (doi: 10.1007/s10211-015-0214-z).
Recent 3 decade-studies on hynobiids have demonstrated that the following 3 life-history traits are common to many species of this family:
(1) Increased head width of the male during the aquatic-breeding phase especially of lentic-breeding hynobiids, i.e., a trait resulted from the swelling of the whole body, is unknown in other families (Hasumi and Iwasawa, 1990: doi: 10.2307/1564217). In case of male-larger "sexual size dimorphism" (SSD: like Hynobius nigrescens), male-male competition is considered to enhance the increased head width (Hasumi, 1994: doi: 10.2307/1564635; Alcorn et al., 2013). In contrast, in case of female-larger SSD (like Salamandrella keyserlingii), the swelling of the whole body of small-sized males, including the increased head width, is hypothesized to develop temporarily during the breeding season as much as large-sized females to resolve intersexual conflict (Hasumi, 2010: doi: 10.1007/s11692-010-9080-9)(for the hypotheses mentioned above, see also Hasumi [2015]: doi: 10.1007/s10211-015-0214-z).
(2) Unlike nonhynobiids, male hynobiids are characterized by the absence of "spermiation" (i.e., sperm release from the testes into the vasa deferentia) during fall and, in northern populations, winter months even after the completion of spermiogenesis (Hasumi et al., 1990: doi: 10.2307/1446342). This leads to a trait of mating unreadiness during fall or winter months in hynobiids. A trait similar to this (i.e., absence of spermiation during fall) has recently been verified also in Salamandrella keyserlingii (Bulakhova and Berman, 2014; Yartsev and Kuranova, 2015). By contrast, Chen et al. (2016) claimed that many species of the genus Hynobius endemic to the mainland of China breed during winter. However, the Chinese salamanders are distributed at low latitudes (southern areas) so that their breeding activities only become earlier than those of others, I think.
(3) Among migratory salamanders (i.e., ambystomatids, hynobiids, and salamandrids) with complex life cycles, hynobiids have unique fall immigration, unrelated to mating, toward terrestrial hibernacula near an aquatic-breeding area (Hasumi and Kanda, 2007: doi: 10.1655/0018-0831(2007)63[163:PAEBMP]2.0.CO;2). This phenomenon has been demonstrated based on the hypothesis that salamander individuals cannot hide themselves within a wetland, where subterranean burrows are saturated with water, and should therefore be captured equally each month if they are year-round residents (Hasumi and Kanda, 2007: doi: 10.1655/0018-0831(2007)63[163:PAEBMP]2.0.CO;2). That is, an extremely high rate of captured individuals in September over three consecutive years reveals their fall immigration to within the wetland. In some of the counterparts of hynobiids examined, such as ambystomatids and salamandrids, mating occurs during fall or winter months immediately after completing immigration to water (e.g., Notophthalmus viridescens: Healy, 1975), except for several northern species or populations where both immigration and mating occur only during spring.
In hynobiid species there had been only anecdotal evidence that salamander individuals utilize subterranean burrows excavated by small mammals as terrestrial refugia, as has been shown in ambystomatid species. That is, it had not practically been confirmed whether hynobiid species use tunnel systems, excavated by other animals, for refuge even if researchers have some speculation that it perhaps does so (a case of Hynobius tokyoensis remains no more than overspeculation at the basis of finding individuals around the entrance of tunnels: Kusano and Miyashita, 1984; T. Kusano, personal communication). Recently, it was confirmed that in Salamandrella keyserlingii at Shaamar, Mongolia, individuals used Muskrat (Ondatra zibethicus) burrows for temporary refuge during daytime in summer (Hasumi et al., 2009: doi: 10.1643/CP-07-237). Also, at Darhadyn, Mongolia, terrestrial individuals of this species are shown to have "aggregation" (i.e., clustering of individuals within a given sex or age class over small intervals of distance) and "site tenacity" (i.e., persistent attachment of individuals to a specific location as a component of territoriality) for the first time in the family Hynobiidae (Hasumi et al., 2014: doi: 10.1007/s00300-013-1443-0).
In this context, hynobiid salamanders are unique among caudate amphibians. Since 52% (47/90 species: Frost, 2022) of hynobiids are endemic to Japan (this percentage had been reduced from the past percentage of approximately 50% due to the increased numbers of the new species in other regions but has returned to this percentage by the new species described in 2018-2019), we Japanese biologists have most advantages around the world for the study of hynobiids. However, almost all of these biologists have not yet been aware of the evolutionary significance of the existence of hynobiids. This unawareness overtly prevents the study of hynobiids from developing in the Japanese scientific societies.
Also, many populations of amphibian species have been adversely affected by human-induced pressures such as development and environmental pollution (e.g., Deformity), and consequently have declined within the last three decades. Among hynobiid species, however, conservation measures have met with limited success due to the absolute lack of our understanding of their life-history strategies (i.e., fundamental knowledge of how they exist). I am afraid of the current of the times that all field scientists should engage in any applied studies regarding environment and conservation, rather than basic studies such as breeding ecology, bebavioral ecology, and evolutionary ecology.
Hence, I recommend you will conduct the study of hynobiids in considering both the evolutionarily known traits (see above) and the newly demonstrated traits mentioned in the section on Study Summary, immediately after visiting the present webpage. I am very happy to hear this page will help elucidate your study goals when dealing with hynobiids.
*On 18 July 2007, I found a mysterious, grey literature reference saying that Salamandrella keyserlingii (Caudata: Hynobiidae) accomplished internal fertilization (Kuranova and Saveliev, 2006). They stated that in the beginning of July, copulation took place and spermatozoa were found in female oviducts (aquatic-breeding season is from April-May). They also stated that females reserved spermatozoa or fertilized eggs in the oviduct until spring. Within that day, for this reference I asked Dr. David M. Sever (Southeastern Louisiana University, USA), a world ultimate expert on the reproduction of amphibians and reptiles (Sever, 2002). His comments were as follows (he permitted me to cite his comments in my webpage):
I am skeptical of their findings. The illustrations of marked sperm in the oviduct are not convincing to me, and the sperm shown in Fig. 5 could be from the testes or sperm ducts. Their findings certainly need confirmation, but if oviductal sperm storage does occur in a hynobiid, that would be most interesting!
I agree with his viewpoint. However, even if oviductal sperm storage occurs in this species, I cannot imagine any courtship behavior of how occurs sperm transfer to females (on land?) in such an external fertilizer that does not produce a spermatophore. Also, I do not understand why they used the term "newt" for this hynobiid species. Although I have reserved referring to the aforementioned questions for a year, I am now confident in myself to submit these questions here.
Recently, Bulakhova and Berman (2014) determined a male reproductive cycle (limited to seasonal changes in testes and vasa deferentia) and an aquatic-breeding behavior of S. keyserlingii and then declined the possibility of terrestrial internal fertilization (Kuranova and Saveliev, 2006). However, in my opinion as a signed reviewer of this paper, showing the reproductive cycle and the breeding behavior was not enough to refute the hypothesis of terrestrial internal fertilization. Also, regrettably, they did not follow my review comment that if they want to decline this hypothesis, they should verify whether or not the male could produce a spermatophore by examining the male's cloacal glands. By the way, like Hynobius nigrescens (Hasumi et al., 1990: doi: 10.2307/1446342), it was confirmed that also in male S. keyserlingii no spermatogenic wave was discernible along the cephalo-caudal axis of the testis (Bulakhova and Berman, 2014).
Although terrestrial internal fertilization is hypothesized in S. keyserlingii (Kuranova and Saveliev, 2006), Yartsev and Kuranova (2015) have concluded that all the reproductive traits of this species suggest that it adopts the external mode of fertilization only, as has been shown in other hynobiid species examined. Incidentally, like H. nigrescens and Hynobius hidamontanus (Hasumi, 1996: Stable URL), it was verified that also in female S. keyserlingii ovarian eggs assumed mint green color in July during the process of yolk accumulation (Yartsev and Kuranova, 2015). Without referring to the ovarian eggs assumed mint green color in H. nigrescens, providing camouflage in algae-laden ponds suggested by Hasumi (1996: introduced by Mathis, 1997), Muto et al. (2017) revealed green algae symbiosis in green-colored eggs of this species.
Alcorn, M. A., J. Deitloff, S. P. Graham, and E. K. Timpe. 2013. Sexual dimorphism in head shape, relative head width, and body size of Eurycea aquatica and Eurycea cirrigera. Journal of Herpetology 47: 321-327.
Arntzen, J. W. 2002. Seasonal variation in sex ratio and asynchronous presence at ponds of male and female Triturus newts. Journal of Herpetology 36: 30-35.
Bulakhova, N. A., and D. I. Berman. 2014. Male reproductive cycle of the Siberian salamander Salamandrella keyserlingii (Caudata: Hynobiidae) in coastal tundra of the Sea of Okhotsk. Polar Biology 37: 123-133.
Chen, C., J. Yang, Y. Wu, Z. Fan, W. Lu, S. Chen, and L. Yu. 2016. The breeding ecology of a critically endangered salamander, Hynobius amjiensis (Caudata: Hynobiidae), endemic to eastern China. Asian Herpetological Research 7: 53-58.
Dowling, H. G., and W. E. Duellman. 1973. Systematic Herpetology: A Synopsis of Families and Higher Categories. HISS Publications, New York, New York, U.S.A.
Duellman, W. E., and L. Trueb. 1986. Biology of Amphibians. McGraw-Hill, New York, New York, U.S.A.
Frost, D. R. 2022. Amphibian Species of the World, an Online Reference. Version 6.1 (Date of access: 28 April 2022). American Museum of Natural History, New York, U.S.A.
Hasumi, M. 1994. Reproductive behavior of the salamander Hynobius nigrescens: monopoly of egg sacs during scramble competition. Journal of Herpetology 28: 264-267.
Hasumi, M. 1996. Seasonal fluctuations of female reproductive organs in the salamander Hynobius nigrescens. Herpetologica 52: 598-605.
Hasumi, M. 2001. Sexual behavior in female-biased operational sex ratios in the salamander Hynobius nigrescens. Herpetologica 57: 396-406.
Hasumi, M. 2010. Age, body size, and sexual dimorphism in size and shape in Salamandrella keyserlingii (Caudata: Hynobiidae). Evolutionary Biology 37: 38-48.
Hasumi, M. 2015. Social interactions during the aquatic breeding phase of the family Hynobiidae (Amphibia: Caudata). Acta Ethologica 18: 243-253.
Hasumi, M., T. Hongorzul, and M. Nakagawa. 2014. Aggregation and site tenacity under downed logs in Salamandrella keyserlingii (Caudata: Hynobiidae). Polar Biology 37: 459-470.
Hasumi, M., T. Hongorzul, and K. Terbish. 2009. Burrow use by Salamandrella keyserlingii (Caudata: Hynobiidae). Copeia 2009: 46-49.
Hasumi, M., and H. Iwasawa. 1990. Seasonal changes in body shape and mass in the salamander, Hynobius nigrescens. Journal of Herpetology 24: 113-118.
Hasumi, M., H. Iwasawa, and Y. Nagahama. 1990. Seasonal dynamics of reproductive organs in male salamanders of the species Hynobius nigrescens. Copeia 1990: 367-377.
Hasumi, M., and F. Kanda. 2007. Phenological activity estimated by movement patterns of the Siberian salamander near a fen. Herpetologica 63: 163-175.
Healy, W. R. 1975. Breeding and postlarval migrations of the red-spotted newt, Notophthalmus viridescens, in Massachusetts. Ecology 56: 673-680.
Kakegawa, M., and M. Hasumi. 2017. Effects of controlled water temperatures on oviposition in a lotic-breeding and externally fertilizing salamander (Hynobius kimurae). River Research and Applications 33: 1036-1043.
Kakegawa, M., and M. Hasumi. 2018. A lotic-breeding salamander (Hynobius kimurae) modifies physiological and morphological traits during wintering. Acta Zoologica 99: 357-366.
Kakegawa, M., F. Kishi, Y. Saikawa, and M. Hasumi. 2017. Seasonal changes in body shape and mass in a lotic-breeding and externally fertilizing salamander Hynobius kimurae. Zoologischer Anzeiger 268: 55-63.
Kuranova, V. N., and S. V. Saveliev. 2006. Reproductive cycles of the Siberian newt Salamandrella keyserlingii Dybowsky, 1870, pp. 73-76. In: Herpetologia Bonnensis II. Proceedings of the 13th Congress of the Societas Europaea Herpetologica. M. Vences, J. Koehler, T. Ziegler, and W. Boehme (eds.). Alexander Koenig Museum, Bonn, Germany.
Kusano, T., and K. Miyashita. 1984. Dispersal of the salamander, Hynobius nebulosus tokyoensis. Journal of Herpetology 18: 349-353.
Larson, A., and W. W. Dimmick. 1993. Phylogenetic relationships of the salamander families: an analysis of congruence among morphological and molecular characters. Herpetological Monographs 7: 77-93.
Mann, T. 1984. Spermatophores. Springer-Verlag, Berlin, Germany.
Mathis, A. 1997. More Herpeto-trivia. HL Comunications (The Newsletter of the Herpetologists' League) 4(1): 7.
Min, M. S., S. Y. Yang, R. M. Bonett, D. R. Vieites, R. A. Brandon, and D. B. Wake. 2005. Discovery of the first Asian plethodontid salamander. Nature 435: 87-90.
Muto, K., K. Nishikawa, R. Kamikawa, and H. Miyashita. 2017. Symbiotic green algae in eggs of Hynobius nigrescens, an amphibian endemic to Japan. Phycological Research 65: 171-174.
Nussbaum, R. A. 1985. The evolution of parental care in salamanders. Miscellaneous Publications of the Museum of Zoology, University of Michigan 169: 1-50.
Paraskiv, K. P. 1953. Semirechensk salamander. Izvestiya Akademii Nauk Kazakhskoi SSR Seiya Biologicheskaya 8: 47-56 (in Russian).
Pough, F. H., R. M. Andrews, J. E. Cadle, M. L. Crump, A. H. Savitzky, and K. D. Wells. 2001. Herpetology, 2nd edition. Prentice-Hall, Upper Saddle River, New Jersey, U.S.A.
Pough, F. H., R. M. Andrews, M. L. Crump, A. H. Savitzky, K. D. Wells, and M. C. Brandley. 2015. Herpetology, 4th edition. Sinauer Associates, Inc., Sunderland, Massachusetts, U.S.A.
Pyron, R. A., and J. J. Wiens. 2011. A large-scale phylogeny of Amphibia including over 2800 species, and a revised classification of extant frogs, salamanders, and caecilians. Molecular Phylogenetics and Evolution 61: 543-583.
Reinhard, S., S. Voitel, and A. Kupfer. 2013. External fertilisation and paternal care in the paedomorphic salamander Siren intermedia Barnes, 1826 (Urodela: Sirenidae). Zoologischer Anzeiger 253: 1-5.
Romer, A. S., and T. S. Parsons. 1977. The Vertebrate Body, 5th edition. W. B. Saunders, Philadelphia, Pennsylvania, U.S.A.
Sever, D. M. 2002. Female sperm storage in amphibians. Journal of Experimental Zoology 292: 165-179.
Thorn, R. 1994. Courtship behavior, fertilization of eggs, and rearing in captivity of the Semirechensk salamander Ranodon sibiricus Kessler (Amphibia, Caudata). Russian Journal of Herpetology 1: 86-90.
Wake, M. H., and R. Dickie. 1998. Oviduct structure and function and reproductive modes in amphibians. Journal of Experimental Zoology 282: 477-506.
Yartsev, V. V., and V. N. Kuranova. 2015. Seasonal dynamics of male and female reproductive systems in the Siberian salamander, Salamandrella keyserlingii (Caudata, Hynobiidae). Asian Herpetological Research 6: 169-183.